Favorite Tips About How To Know If Polar Or Nonpolar
Polar Or Nonpolar Lewis Structure
Unlocking the Secrets of Molecular Personalities
Ever wondered why oil and water just don't mix? Or why some substances dissolve so easily in water, while others stubbornly refuse? The answer, my friend, lies in the fascinating world of molecular polarity. It's all about how molecules share (or don't share!) their electrons, giving them unique electrical "personalities." Think of it like introverts and extroverts—some are happy keeping to themselves (nonpolar), while others are all about connection and interaction (polar). So, how do we figure out which is which? Let's dive in!
1. A Quick Dive into Molecular Bonds
Before we even think about polarity, we need a tiny refresher on chemical bonds. Remember those days in chemistry class? Atoms join together to form molecules by sharing electrons. When atoms share electrons equally, like two equally strong friends tugging on a rope, we get a nonpolar bond. But when one atom is a bit greedier for electrons than the other, it pulls the electron cloud closer, creating a polar bond.
Imagine a tug-of-war where one side is significantly stronger. They'll pull the rope, and the center point, much closer to their side. That's like a polar bond! This uneven sharing creates a slightly negative charge (-) on the more electron-hogging atom and a slightly positive charge (+) on the other. These partial charges are crucial for understanding how molecules interact.
These differences in electronegativity (that's the electron-greediness of an atom) are key. Oxygen, for instance, is a notorious electron hog. If it's bonded to hydrogen, you can bet it's creating a polar bond. Carbon and hydrogen, on the other hand, are more even-keeled, resulting in a relatively nonpolar bond.
Think of it this way: if the electronegativity difference is small, the bond is nonpolar. If the difference is significant, the bond is polar. We're building up the knowledge, piece by piece, like putting together a molecular puzzle!

Polarity Of A Molecule Explained
Spotting Polarity
Alright, now comes the fun part! How do you, as a budding molecular detective, actually determine if a molecule is polar or nonpolar? There are a few key clues to look for.
2. Clue #1
First, examine the individual bonds within the molecule. Are there any highly electronegative atoms bonded to less electronegative ones? Look for things like oxygen, nitrogen, fluorine, or chlorine bonded to carbon or hydrogen. These are your prime suspects for polar bonds.
Remember that electronegativity difference we talked about? There are scales that quantify this, but honestly, you don't need to memorize them. Just knowing that oxygen, nitrogen, and the halogens are generally more electronegative than carbon and hydrogen is a great start. If you see them hanging out together in a bond, chances are it's polar.
It's like spotting a potential argument brewing. If you see two people with very different personalities and opinions, you know there's a higher chance of conflict. Similarly, if you see two atoms with drastically different electronegativities bonded together, the bond is likely polar.
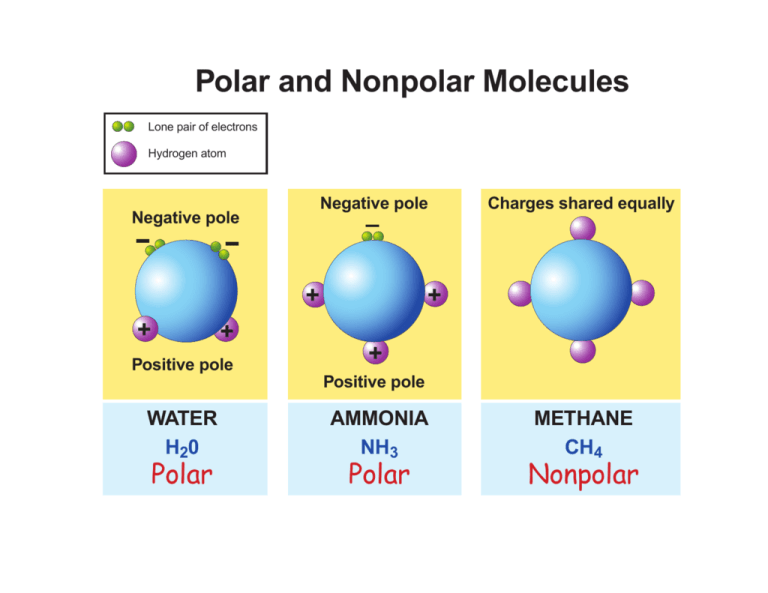
For example, in a molecule of water (H2O), the oxygen atom is significantly more electronegative than the hydrogen atoms. This creates two polar bonds, pointing from the hydrogen atoms toward the oxygen atom. This is the first hint that water is a polar molecule!
3. Clue #2
Even if a molecule contains polar bonds, it doesn't automatically mean the entire molecule is polar. This is where the molecular shape comes into play. The arrangement of atoms in 3D space is absolutely crucial. Think of it as the overall "vibe" of the molecule. Some shapes can cancel out the individual bond polarities, while others amplify them.
If the polar bonds are arranged symmetrically around the central atom, their dipole moments (the direction and magnitude of the polarity) can cancel each other out. Imagine two people pulling equally hard on opposite sides of a box—the box doesn't move. Similarly, if the bond dipoles cancel, the molecule is nonpolar overall.
Carbon dioxide (CO2) is a classic example. It has two polar carbon-oxygen bonds, but the molecule is linear. The two bond dipoles point in opposite directions and perfectly cancel each other out, making CO2 a nonpolar molecule. Mind. Blown.
However, if the polar bonds are arranged asymmetrically, their dipole moments add up, resulting in a net dipole moment for the molecule. This makes the entire molecule polar. Think of water again: its bent shape prevents the bond dipoles from canceling, resulting in a polar molecule. The shape of water, and therefore its polarity, is essential for life as we know it.
4. Clue #3
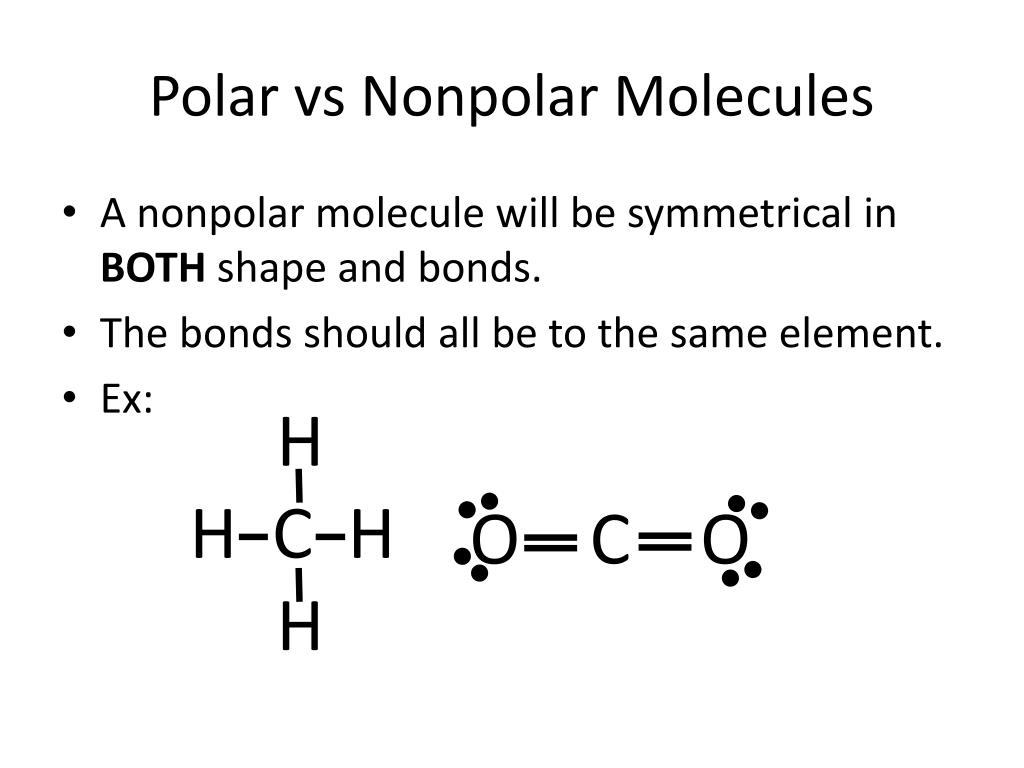
This is essentially a simplification of shape. Look for symmetry. If the molecule is symmetrical, and all the atoms surrounding the central atom are identical, it's likely nonpolar, even if the individual bonds are polar (like in CO2). If there's any asymmetry, or if the atoms surrounding the central atom are different, it's likely polar.
Consider carbon tetrachloride (CCl4). It has four polar carbon-chlorine bonds. However, the molecule has a tetrahedral shape, which is highly symmetrical. Because of this symmetry, the bond dipoles cancel each other out, and CCl4 is a nonpolar molecule. A perfect example of molecular teamwork resulting in overall neutrality!
Ammonia (NH3), on the other hand, has three polar nitrogen-hydrogen bonds and a lone pair of electrons on the nitrogen atom. This lone pair creates asymmetry in the molecule's shape (it's pyramidal), preventing the bond dipoles from completely canceling. As a result, ammonia is a polar molecule.
It's like building a tower. If the base is perfectly square and all the sides are equal, it's a stable, symmetrical structure. But if one side is shorter than the others, the tower becomes asymmetrical and prone to tipping. Molecular symmetry works in a similar way, determining the overall polarity of the molecule.

Understanding Types Of Chemical Bonds TEAS NurseHub
Polarity in Action
So, you've mastered the art of spotting polar and nonpolar molecules. Great! But why should you care? Because polarity dictates a molecule's physical and chemical properties, influencing everything from its boiling point to its ability to dissolve other substances. It's like understanding a person's personality traits—it helps you predict how they'll behave in different situations.
5. Solubility
One of the most important consequences of polarity is its effect on solubility. The general rule is "like dissolves like." Polar solvents (like water) tend to dissolve polar solutes (like salt or sugar), while nonpolar solvents (like oil or hexane) tend to dissolve nonpolar solutes (like fats or waxes). This is because polar molecules are attracted to each other through dipole-dipole interactions and hydrogen bonding, while nonpolar molecules interact through weaker London dispersion forces.
Think of it like magnets. If you have two magnets with opposite poles, they attract each other strongly. Similarly, polar molecules are attracted to other polar molecules. But if you try to stick a magnet to a piece of wood, it won't work because wood doesn't have a magnetic charge. Similarly, polar molecules don't interact well with nonpolar molecules.
This principle explains why oil and water don't mix. Water is highly polar, while oil is primarily nonpolar. They simply don't have any attractive forces between them, so they separate into distinct layers. The polar water molecules prefer to stick together, and the nonpolar oil molecules prefer to stick together.
This is also why some vitamins are water-soluble (like vitamin C) and others are fat-soluble (like vitamin A). Water-soluble vitamins can dissolve in the watery environment of the body, while fat-soluble vitamins can dissolve in the fatty tissues. Understanding polarity is crucial for understanding how nutrients are absorbed and transported in the body!
6. Boiling Points
Polarity also affects a substance's boiling point. Polar molecules have stronger intermolecular forces (the attractions between molecules) than nonpolar molecules. These stronger forces require more energy to overcome, resulting in higher boiling points.
Imagine trying to separate a group of people who are holding hands tightly versus a group of people who are barely touching each other. It's much harder to pull apart the group that's holding hands tightly. Similarly, it takes more energy (higher temperature) to separate polar molecules that have strong intermolecular forces.
Water, with its strong hydrogen bonding due to its polarity, has a relatively high boiling point compared to other molecules of similar size. This is essential for life because it allows water to exist as a liquid at temperatures that support biological processes. If water were nonpolar, it would boil at a much lower temperature, and life as we know it wouldn't be possible!
Even within a group of molecules with similar molecular weights, the more polar molecules will generally have higher boiling points. The strength of the intermolecular forces, dictated by polarity, is a major factor in determining how easily a substance transitions from a liquid to a gas.

Difference Between Polar And Nonpolar
Putting it all Together
Let's cement this knowledge with a few practical examples. Consider these molecules and see if you can predict their polarity based on what you've learned.
7. Example 1
Methane consists of a central carbon atom bonded to four hydrogen atoms. The carbon-hydrogen bond is considered to be mostly nonpolar because the electronegativity difference between carbon and hydrogen is small. The molecule has a tetrahedral shape, which is highly symmetrical. Therefore, methane is a nonpolar molecule. This explains why methane is a gas at room temperature and doesn't dissolve in water.
Think of methane as a group of friends who are all equally indifferent to each other. They don't have strong attractions or repulsions, so they're happy to float around and do their own thing. That's why methane is a gas!
You'll find methane as the primary component of natural gas, used for heating homes and generating electricity. Its nonpolar nature allows it to exist as a gas at ambient temperatures and pressures.
Because it is nonpolar it will dissolve in other nonpolar solvents like oil or hexane, not water.
8. Example 2
Ethanol contains both polar and nonpolar regions. The ethyl group (CH3CH2) is mostly nonpolar, similar to methane. However, the hydroxyl group (OH) is highly polar due to the electronegativity difference between oxygen and hydrogen. This polar hydroxyl group makes ethanol capable of forming hydrogen bonds with water, making it miscible (soluble) in water.
Ethanol is like a person who's both introverted and extroverted. The ethyl group is the introverted part that prefers to keep to itself, while the hydroxyl group is the extroverted part that loves to interact with others (especially water molecules!).
That mixture of polar and nonpolar regions allows ethanol to dissolve both polar and nonpolar substance and is also used as a solvent for wide range of application.
The polarity of ethanol is directly connected to its uses as an antiseptic, a solvent, and even as a component in alcoholic beverages.
9. Example 3
Benzene is a cyclic hydrocarbon with six carbon atoms arranged in a ring, each bonded to one hydrogen atom. All the bonds within benzene are either carbon-carbon or carbon-hydrogen, both of which are relatively nonpolar. The molecule is also perfectly symmetrical. This makes benzene a nonpolar molecule.
Picture a perfect circle, each point equidistant from the center. That's the symmetry of benzene. And because all its bonds are relatively nonpolar, it's a truly nonpolar molecule through and through.
Benzene's nonpolar nature has implications for its uses as a solvent and its behavior in the environment. And because it can dissolve nonpolar substances it is a good choice for dissolving rubber, paint or ink.
It is important to understand that benzene is a known carcinogen and thus should be handled with extreme caution.

Polar And Nonpolar Periodic Table
FAQs
Still got questions swirling around in your head? No worries! Here are a few frequently asked questions to help clear things up.
10. Q
A: Nope! This is a common misconception. A molecule can have polar bonds, but if the molecule is symmetrical and the bond dipoles cancel each other out, the molecule is nonpolar overall. Think of CO2 as the prime example.
11. Q
A: Water is significantly more polar than oil. That's why they don't mix! Water's bent shape and highly electronegative oxygen atom create a strong dipole moment, making it a polar solvent. Oil, on the other hand, is composed primarily of nonpolar hydrocarbons.
12. Q
A: Absolutely! Polarity can influence how a molecule interacts with the olfactory receptors in your nose. Polar molecules tend to have different smells than nonpolar molecules, and the strength of the smell can also be affected by polarity. But, it's not the only factor — molecular size and shape also play roles.
13. Q
A: Yes! Like ethanol! Some molecules have regions that are polar and regions that are nonpolar. These are often referred to as amphiphilic molecules. They can interact with both polar and nonpolar substances, making them useful in applications like detergents and emulsifiers.