Recommendation Info About Is Battery A Electric Potential

Unlocking the Secrets of Batteries and Electric Potential
1. So, what's the buzz about batteries and electric potential?
Ever wondered what's really going on inside that little powerhouse we call a battery? It's more than just some chemical reactions magically creating electricity. At its heart, the battery operates on the principle of electric potential, sometimes called voltage. Think of it like this: a battery creates an 'electrical hill,' and electrons want to roll downhill, creating a current. This 'hill' is the electric potential. Is the battery itself the electric potential? Well, not exactly, but it's the source of it.
Imagine a water tower. The water at the top has potential energy because it can flow down. The battery is like that water tower, and the electric potential is the height of the water. The higher the tower (higher the voltage), the more potential the water (electrons) has to do work as it flows. Pretty neat, huh?
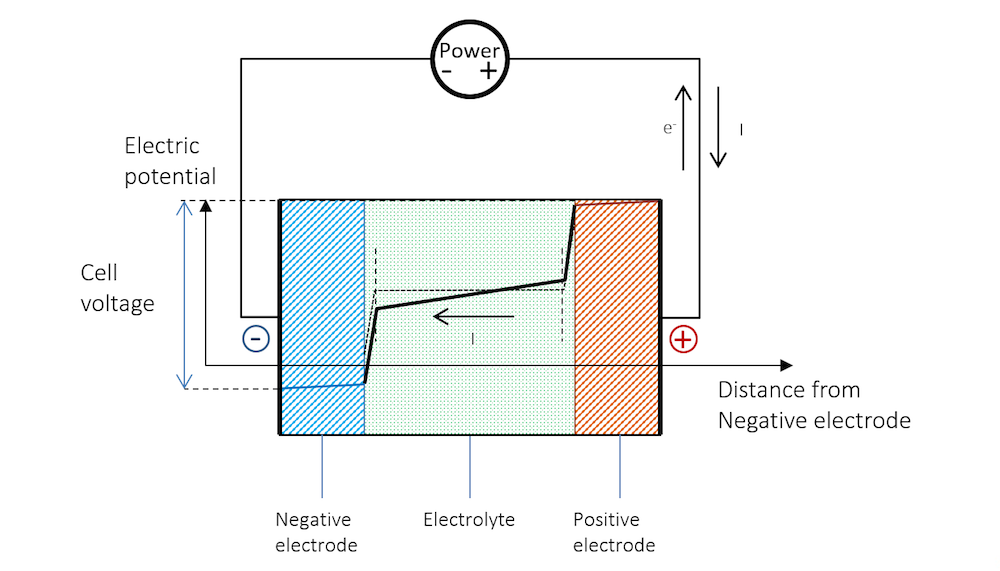
The electric potential isn't actually the battery. It's the difference in electrical potential energy between two points. Inside the battery, chemical reactions separate charges, creating a surplus of electrons at one terminal (the negative one) and a deficit at the other (the positive one). This separation is what establishes the electric potential difference. When you connect a circuit to the battery, those electrons are eager to move from the negative terminal to the positive terminal to balance things out. This flow of electrons is what powers your devices.
So, in simple terms, the battery doesn't become the electric potential; it creates the electric potential difference that allows electrons to flow and do work. It's the engine that drives the electrical current. Think of it as the battery being the baker and the electric potential being the freshly baked pie delicious and ready to be consumed!
What Is A Battery How Does It Work At Ricky Crider Blog
The Electric Potential Difference
2. Digging deeper into the concept.
Let's get a bit more specific. The term "electric potential difference" is really just a fancy way of saying "voltage." It's the amount of work required to move a unit of electric charge from one point to another in an electric field. And that's precisely what a battery does! It creates that electric field by separating charges.
Think about it like pushing a cart uphill. The steeper the hill, the more effort you need to push the cart. Similarly, the higher the voltage (the 'steeper' the electric potential hill), the more energy the electrons have to do work as they flow through a circuit. That's why a 12-volt battery can power more demanding devices than a 1.5-volt battery.
The voltage is measured in volts (V), named after Alessandro Volta, the inventor of the voltaic pile, an early form of battery. A volt is defined as one joule of energy per coulomb of charge (1 V = 1 J/C). So, a 1-volt battery can deliver one joule of energy for every coulomb of charge that flows through it. Are you still with me? Don't worry, we are getting to the "meat" of this topic.
A car battery, often 12V, has a bigger "hill" for the electron to travel down than an AA battery, which is only 1.5V. That is why it can power your car's engine and lights. Different devices require different voltages to operate properly. Using a battery with too low a voltage might not provide enough power, while using one with too high a voltage can damage the device.
Inside a Battery
3. What happens within a battery that allows for electrical potential?
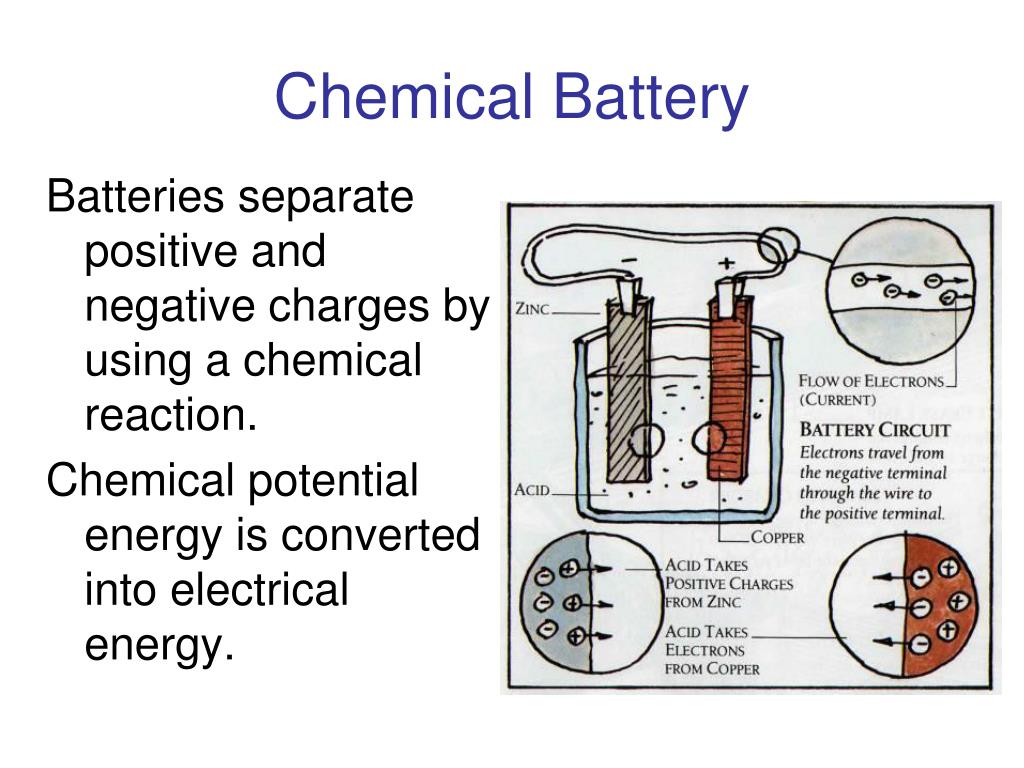
Okay, so we know batteries create electric potential. But how do they actually do that? It all comes down to chemistry, specifically electrochemical reactions. Inside a battery, there are two electrodes (a positive electrode and a negative electrode) immersed in an electrolyte. The electrolyte is a substance that allows ions to move between the electrodes.
At the negative electrode (anode), a chemical reaction occurs that releases electrons. These electrons accumulate at the negative terminal. At the positive electrode (cathode), another chemical reaction occurs that consumes electrons. This creates a deficit of electrons at the positive terminal. Its this difference in electron concentration — the electric potential difference — that drives the current when you connect a circuit.
Different types of batteries use different chemical reactions. For example, a common alkaline battery uses the reaction between zinc and manganese dioxide. A lithium-ion battery, commonly used in smartphones and laptops, uses lithium compounds. The specific chemical reactions determine the voltage and energy capacity of the battery.
When the chemical reactions inside the battery are complete (i.e., all the reactants are used up), the battery is "dead" because it can no longer maintain the electric potential difference. Rechargeable batteries can have their chemical reactions reversed by applying an external voltage, restoring the battery's ability to deliver power. But that is a topic for another day!

What Is The Potential Difference Across Battery In Electric
Beyond the Battery
4. Where else do we see electrical potential?
The concept of electric potential isn't limited to just batteries. It's fundamental to understanding how electricity works in general. Electric potential differences exist wherever there are separated charges, whether it's in a lightning storm, a power outlet, or even the neurons in your brain!
Consider a power outlet in your wall. The power company maintains an electric potential difference between the two slots (typically 120 volts in North America). When you plug in an appliance, you're creating a circuit that allows electrons to flow from one slot to the other, powering the appliance. The power plant acts as a giant 'battery' maintaining this electric potential difference across the power grid.
Even lightning is an example of electric potential in action! Charge builds up in clouds due to friction between ice crystals and water droplets. When the electric potential difference between the cloud and the ground becomes large enough, a massive discharge of electrons occurs, creating a visible spark of lightning. And you thought that was simply a trick of the light!
On a smaller scale, the electric potential plays a crucial role in the functioning of your nervous system. Neurons use electric potential differences across their cell membranes to transmit signals throughout your body. These signals are what allow you to think, move, and perceive the world around you. Electric potential is truly everywhere we look!

Debunking Myths and Common Misconceptions
5. Setting the record straight.
One common misconception is that batteries "store" electricity. While it's a convenient way to think about it, it's not entirely accurate. Batteries store chemical energy, which they convert into electrical energy by creating an electric potential difference. The electricity itself is the flow of electrons driven by this potential.
Another myth is that all batteries of the same voltage deliver the same amount of power. Voltage is only one factor. The amount of current a battery can deliver (measured in amperes or amps) is equally important. A battery with a higher amp rating can deliver more power, even if it has the same voltage as a battery with a lower amp rating. Think of it as voltage being the pressure of the water and amperage being the flow rate. You need both for effective work!
Some people also believe that leaving a battery in a device when it's not being used will automatically drain the battery. While some devices do have a small "standby" current that can slowly drain the battery, this is not always the case. Modern devices are generally designed to minimize standby current. That being said, it's still a good idea to remove batteries from devices you won't be using for an extended period, as they can leak over time.
Finally, never throw batteries into a fire! Batteries contain hazardous materials that can be released into the environment when burned. Always dispose of batteries properly at a designated recycling center. Your planet will thank you.
Electric Current Battery Diagram
FAQ
6. Let's wrap up with some answers to frequent questions.
Still got questions about batteries and electric potential? No problem! Here are a few frequently asked questions:
Q: Does a higher voltage mean a stronger battery?
A: Not necessarily. Higher voltage means a greater potential to push electrons through a circuit, but the battery's overall "strength" (how long it lasts) also depends on its capacity to deliver current (measured in amp-hours or milliamp-hours).
Q: Can I mix different types of batteries in a device?
A: It's generally not recommended. Different battery types have different voltage characteristics and discharge rates. Mixing them can lead to uneven discharge, reduced performance, and even potential damage to the device or the batteries themselves.
Q: What does mAh mean on a battery?
A: mAh stands for milliampere-hour. It's a measure of the battery's capacity — how much current it can deliver over a certain period of time. A battery with a higher mAh rating will generally last longer than one with a lower mAh rating, assuming they're used in the same device.
Q: Why do batteries eventually die, even when not in use?
A: Batteries naturally self-discharge over time. Internal chemical reactions continue to occur even when the battery isn't connected to a circuit, slowly depleting its stored energy. The rate of self-discharge varies depending on the battery type and environmental conditions (like temperature).
Q: Are there batteries that never need charging?
A: No. All chemical batteries will eventually run out of energy and need to be replaced or recharged. There are some devices that are able to generate electricity via solar, nuclear or other means, but they're not typically "batteries" in the traditional sense.