Outstanding Info About Which Buffer Is More Effective

Decoding the Buffer Mystery
1. Understanding Buffers
So, you're wading into the world of buffers, huh? Don't worry, it sounds more intimidating than it is. Think of a buffer as a temporary holding zone. Like a waiting room for data, or a strategic reserve of alkalinity in a chemical solution. In the tech realm, it prevents data loss or lag. In chemistry, it keeps pH levels stable. Both very important jobs, if you ask me.
We're not talking about polishing your shoes here, but rather about preventing digital or chemical chaos. Different buffers exist for different purposes, and knowing which one to use can be the difference between smooth sailing and a complete meltdown. It's like choosing the right tool for the job — a hammer won't help you screw in a lightbulb, and a phosphate buffer might not be the best choice for your biological experiment. Speaking of which...
The effectiveness of a buffer depends entirely on the context. Are we talking about video streaming, audio recording, or maybe titrating a solution in the lab? The answer will change depending on these parameters. The key is to match the buffer characteristics with the specific needs of the application. For example, you wouldn't use a network buffer designed for high-speed data transfer to stabilize the pH of a delicate cell culture. It's all about finding the perfect fit, kind of like finding the perfect pair of jeans — comfy and functional!
Ultimately, no single buffer is universally "more effective." It's all relative! And that's why it is important to dive into some specifics, considering the diverse range of scenarios where buffers play crucial roles.

Chemical Buffers
2. Peeking into the World of pH Balance
Let's get our lab coats on (metaphorically, of course — unless you actually are in a lab). Chemical buffers are substances that resist changes in pH when small amounts of acid or base are added. Imagine them as tiny pH bodyguards, constantly working to maintain equilibrium. They are a blend of a weak acid and its conjugate base or a weak base and its conjugate acid. This dynamic duo acts as a chemical seesaw, balancing out the incoming acid or base to minimize pH fluctuations.
Think about your blood. It needs to maintain a pretty stable pH (around 7.4) for everything to work correctly. Buffers in your blood, like the bicarbonate buffering system, help prevent drastic pH shifts that could be life-threatening. If the blood becomes too acidic (acidosis) or too alkaline (alkalosis), this can cause major disruptions of enzyme structure, protein structure, and overall bodily function. Therefore, our body use a buffer solution to maintain the pH of the blood.
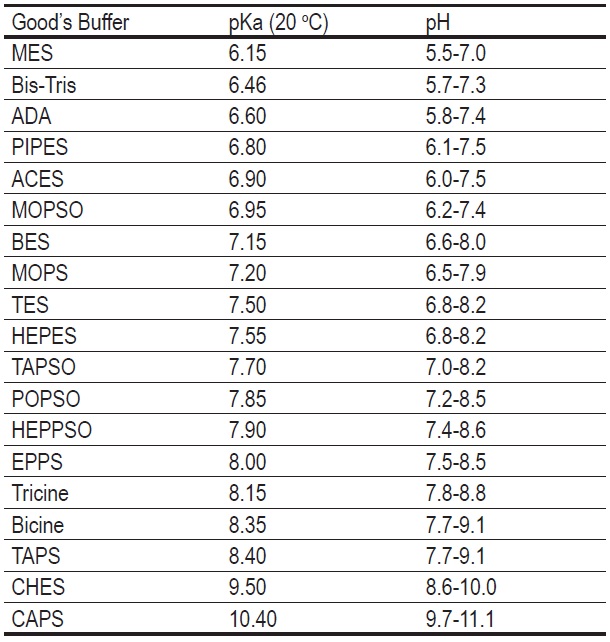
Different chemical buffers work best at different pH ranges. Acetic acid buffers are great for slightly acidic solutions, while Tris buffers are commonly used for more alkaline conditions. The choice depends on the pH you're trying to maintain. It's like choosing the right paint color — you wouldn't use bright yellow in a dimly lit room, would you?
One factor affecting the effectiveness of a buffer is its buffer capacity. This refers to the amount of acid or base the buffer can neutralize before its pH changes significantly. A higher buffer capacity means it can handle more "attacks" on its pH without faltering. Selecting a buffer with an appropriate buffer capacity is crucial for maintaining a stable pH environment in your experiment or system.

Digital Buffers
3. Delving into the Digital Realm
Now, let's trade our lab coats for hoodies and dive into the digital world! In the realm of computers and electronics, buffers take on a different form. Here, a buffer is a region of memory used to temporarily hold data while it is being moved from one place to another. Think of it like a staging area for information, ensuring a smooth and uninterrupted flow. This is particularly vital when the data source and destination operate at different speeds or when dealing with asynchronous processes.
Imagine streaming a video online. The video data is downloaded in chunks, but your computer needs to display it smoothly, without stuttering. A buffer stores some of the incoming data, allowing your computer to play the video at a consistent rate, even if your internet connection fluctuates a bit. Without a buffer, you'd be stuck with constant buffering, and nobody wants that!
Different types of digital buffers exist, each optimized for specific applications. Some are designed for high-speed data transfers, while others are focused on minimizing latency (delay). Network buffers, for instance, are used to manage the flow of data packets across a network, preventing congestion and ensuring reliable communication. Video buffers are tuned to handle the large amounts of data required for smooth video playback.
The size of a digital buffer is also a crucial factor. A buffer that's too small can lead to data loss or performance bottlenecks, while a buffer that's too large can waste memory. It's a balancing act, finding the sweet spot that ensures smooth operation without excessive resource consumption. Much like finding the perfect amount of coffee to keep you awake without the jitters!
Good's Buffer Tricine DOJINDO
Comparing Apples and Oranges
4. The Nuances of Effectiveness
So, can we directly compare chemical buffers and digital buffers? Not really. They serve fundamentally different purposes in vastly different contexts. It's like comparing apples and oranges — both are fruits, but they have different tastes, textures, and nutritional profiles. Similarly, both chemical and digital buffers act as stabilizers, but they operate on completely different principles and address different challenges.
The "effectiveness" of a chemical buffer is measured by its ability to resist pH changes, while the "effectiveness" of a digital buffer is measured by its ability to prevent data loss and ensure smooth data flow. You wouldn't use a digital buffer to neutralize an acid, and you wouldn't use a chemical buffer to stream a video. They are tools for different jobs!
However, both types of buffers share some common underlying principles. Both rely on a sort of "reserve capacity" — a chemical buffer has a reserve of acid or base, while a digital buffer has a reserve of memory. Both work to smooth out fluctuations and maintain a stable state. In both cases, matching the buffer's characteristics to the specific application is crucial for optimal performance.
Ultimately, understanding the specific requirements of your application is key to choosing the right buffer. Whether you're a chemist, a computer scientist, or just someone curious about the world around you, knowing the difference between these types of buffers can help you navigate the complexities of your field. Buffers are just another tool, but a good tool will always make work easier.

Buffer Solution Definition, Types, Formula, Examples, And, 53 OFF
Putting it all together
5. Making Informed Decisions
Choosing the "more effective" buffer is, at its core, a matter of understanding the specific needs of the system you're working with. Just as a seasoned chef wouldn't use the same knife for every ingredient, so too must you tailor your buffer selection to the task at hand. Factors such as the desired pH range for a chemical reaction, the data transfer rate for a digital system, and the tolerance for fluctuations all play crucial roles in determining which buffer will deliver optimal results.
Think about it: in a laboratory setting, the precise control of pH can be paramount for the success of an experiment. Enzymes, for example, often have very narrow pH ranges within which they function optimally. Selecting the appropriate chemical buffer with the right buffering capacity becomes a critical consideration, ensuring that the reaction environment remains stable and conducive to the desired outcome. Using the incorrect buffer in such a scenario could lead to inaccurate results or even complete failure of the experiment.
On the digital front, considerations such as bandwidth, latency requirements, and data integrity take center stage. A video streaming service, for instance, might employ a sophisticated buffering system to ensure smooth playback, even when network conditions fluctuate. Factors such as buffer size, buffering algorithms, and error correction mechanisms all contribute to the overall effectiveness of the system. Choosing a buffer that can effectively manage these challenges is crucial for delivering a seamless user experience.
In conclusion, there's no universal "best" buffer. The "more effective" buffer is always the one that's best suited to the specific task at hand. It demands a thoughtful assessment of the underlying needs of a given system, considering factors such as stability, responsiveness, and resource utilization. By understanding the nuances of both chemical and digital buffers, you can make informed decisions that lead to optimal performance, whether you're conducting cutting-edge research in the lab or building the next generation of digital applications.

How Do Buffers Work? With Buffer Calculations! AP Chemistry Unit 8.3
Frequently Asked Questions (FAQs)
6. What is a buffer capacity, and why is it important?
Buffer capacity refers to the amount of acid or base a buffer can neutralize before experiencing a significant pH change. It's crucial because it determines how well the buffer can maintain a stable pH in the face of external disturbances. A higher buffer capacity means the buffer can resist pH changes more effectively.
7. Can I use any buffer for any pH range?
No. Each buffer has an optimal pH range where it works best. Using a buffer outside its effective range will result in poor buffering capacity and unstable pH.
8. Are digital buffers always necessary?
Not always, but they are often crucial in situations where data transfer rates are uneven or where real-time performance is critical. Without a buffer, data loss or interruptions are more likely.
9. How do I choose the right chemical buffer for my experiment?
Consider the desired pH range, the compatibility of the buffer with other components in your system, and its buffering capacity. Consulting reference tables and literature can also be helpful.